3rd Generation Lentiviral Packaging Plasmid Mix
Product Description
For the production of lentiviral particles, three components are generally required: 1) a lentiviral vector containing your inserts of interest (cDNA, shRNA, or miRNA), 2) one or two packaging vectors which contain all necessary viral structure proteins, 3) an envelope vector expressing Vesicular Stomatitis Virus (VSV) glycoprotein (G).
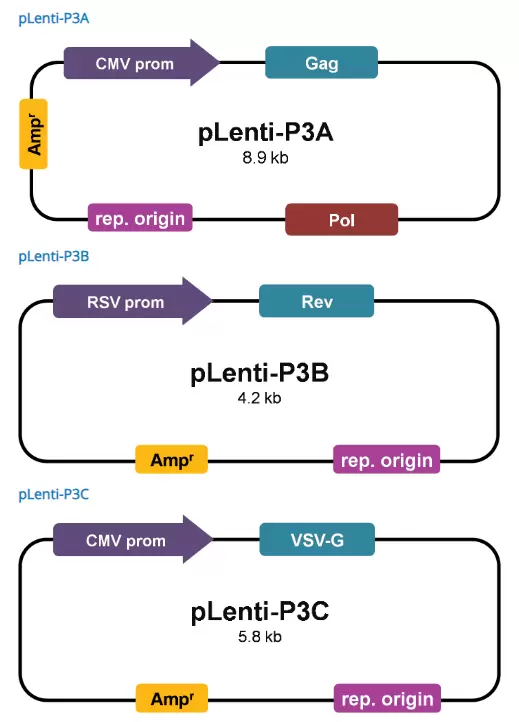
The 3rd generation packaging system offers maximal biosafety as the lentiviral Rev gene is supplied as an independent vector from other structure genes, further eliminating the possibility of reverse recombination of vectors into replication competent viral particles.
The 3rd generation lentiviral packaging mix will only support lentiviral expression vectors with a chimeric 5' LTR in which the HIV promoter is replaced with CMV or RSV, thus making it TAT-independent. The 3rd generation lentiviral vectors will not support the production of 2nd generation lentiviral particle productions. All ABM lentiviral vectors are TAT-independent.
Components of the Mix
(200ul mix of 3 plasmids)
Selection: Ampicillin (Bacterial Selection)
Amount: 200 μl (100 µg mixture of pLenti-P3A, pLenti-P3B, and pLenti-P3C)
Appearance: Liquid
Storage: Temperature °20-C or below (shipped at ambient temperature)
Shelf Life: Up to 1 year (when at °20-C or below in a frost-free freezer)
Product Citations
- Chan, C.-H., Chiou, L.-W., Lee, T.-Y., Liu, Y.-R., Hsieh, T.-H., Yang, C.-Y., & Jeng, Y.-M. (2022) PAK and PI3K pathway activation confers resistance to KRASG12C inhibitor sotorasib British Journal of Cancer
- Shirasaki, T., González-López, O., McKnight, K. L., Xie, L., Shiota, T., Chen, X., Feng, H., & Lemon, S. M. (2022) Nonlytic Quasi-Enveloped Hepatovirus Release Is Facilitated by pX Protein Interaction with the E3 Ubiquitin Ligase ITCH Journal of Virology
- Lai, T. H., Ahmed, M., Hwang, J. S., Zada, S., Pham, T. M., Elashkar, O., & Kim, D. R. (2021) Transcriptional Repression of Raf Kinase Inhibitory Protein Gene by Metadherin during Cancer Progression International Journal of Molecular Sciences
- Catalog Number
LV053-ABM - Supplier
ABM - Size
- Shipping
Blue Ice

