Adeno-Associated Virus (AAV) Service abm
Comprehensive AAV Production Service
Product Description
abm offers a Custom AAV Service including design, clone and/or viral package your custom AAV construct for a range of AAV serotypes (1-11, DJ, DJ8). Purifications include crude (ideal for in vitro experiments) and purified (optimal for in vivo experiments). Inquire with us if you are looking for Custom Cloned AAV Vectors or Custom AAV Packaging or both. You can also send your own vector.
Choose between 3 variants of Custom AAV Production
- Custom AAV Production without DNA Amplification
- Custom AAV Production with DNA Amplification
- Custom siRNA AAV Production
Generation of rAAV vectors requires 4 key components: plasmids acting in-trans and the transgene acting in-cis. These components include:
- a plasmid containing the AAV Rep and Cap genes required for capsid formation and replication,
- a plasmid containing the necessary adenovirus helper genes,
- a cassette containing the transgene enclosed by two inverted terminal repeats (ITR), and
- a viral packaging cell line
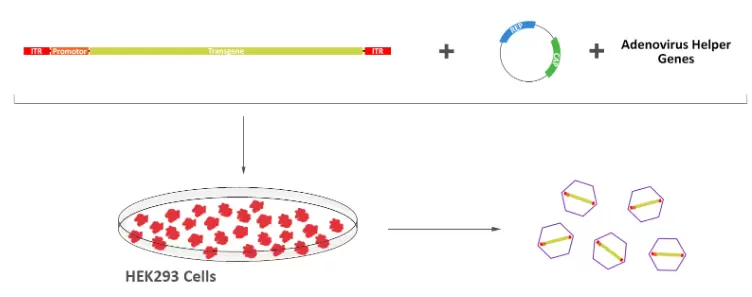
Fig.1. Depiction of the modern rAAV production protocol. In the modern system, Ad Helper Virus is replaced with only their essential genes required for rAAV packaging. This system completely eliminates adenovirus contamination in rAAV productions and simplifies the purification step.
The table below shows a summary of the natural tropisms and recommended applications of the common AAV serotypes:
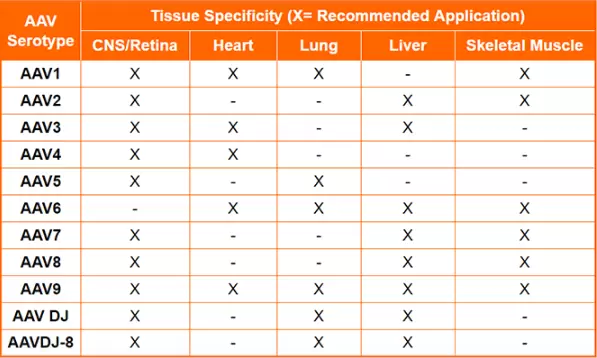
Still unsure which serotype to choose for your experiment? The AAV Serotype Blast™ Kit is ideal for testing the infection efficiency of the different AAV serotypes in different cell types. The kit contains premade GFP-expressing AAV in serotypes 1 - 9.
Performance Data
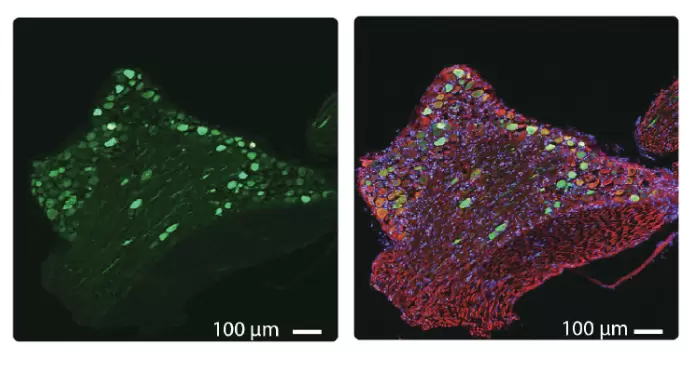
Fig.2 AAV infectivity Data. EGFP expression in lumbar neuronal cells 4 weeks after intrathecal injection into mice (Left), and seen with β-tubulin (red) and DAPI (blue) overlay (Right). Courtesy of Dr. D Lopes, King's college London.
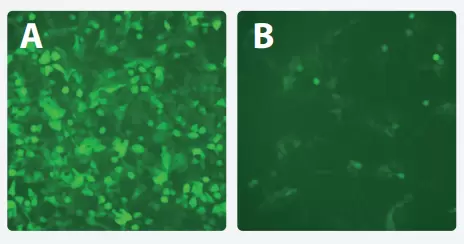
Figure 2. abm’s Adeno-associated viruses (AAV) outperform competitors in transduction eciency at both the same serotype and viral titer. HEK293 cells were infected with 2μl of a 1013GC/ml AAV-GFP virus (serotype 8) from either abm (A) or a competitor (B). Images were taken 24 hours post-infection.
Further services are available on request, please get in touch with us:
- Custom AAV Titration
- AAV Virus Custom Aliquoting
Genetically Modified Organism (GVO)
See General Terms.
- Catalog Number
CS-AAV-Production-GVO-ABM - Supplier
ABM - Size
- Shipping
Dry Ice
- Price
- Please inquire

