AAVancedTM Concentration Reagent (250 ml aliquot)
| Specifications | |
|---|---|
| Product Category: | AAV Purification |
Product Description
One-step rAAV particle isolation from packaging cell media
Traditional rAAV production methods requires multiple steps, including cell lysis and CsCl2 ultra-high-speed density gradient centrifugation, chromatography, or binding to affinity matrix columns. These are difficult to setup, time-consuming, and require specialized equipment for isolation of high-purity rAAV for in vivo experiments. As many studies have shown that different rAAV serotypes are efficiently secreted into the culture medium of transfected 293T cells during rAAV packaging, SBI has developed a novel reagent to facilitate fast, high-quality rAAV isolation.
Easiest Protocol to Isolate rAAV Particles
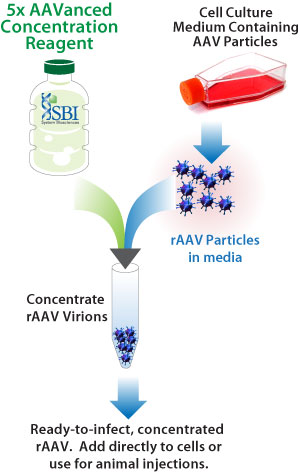
SBI has developed an innovative solution called AAVanced Concentration Reagent - a simple, one step rAAV concentration reagent for the isolation of rAAV particles from media. AAVanced Concentration Reagent is based on a proven nanoparticle technology and is specifically optimized for the precipitation of most AAV serotypes from culture medium. The rAAV virus produced using AAVanced Concentration Reagent has been tested successfully for in vitro and in vivo applications for multiple serotypes of AAV with no observed cytotoxic effects, which provides direct evidence for the utility of this reagent for demanding applications using rAAV. SBI's AAVanced Concentration Reagent significantly reduces the complexity and time required for rAAV virus production, allowing researchers to focus on their research experiments rather than virus production.
AAVanced Concentration Reagent Produces Robust Virions
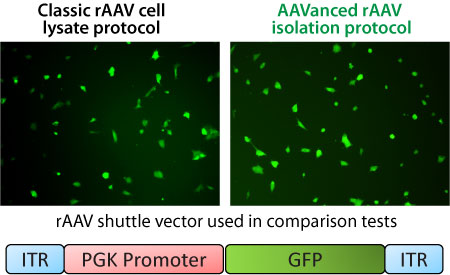
How does AAVance virus preps compare to tranditonal cell lysate preps?
To test this comparison, recombinant rAAV was packaged with a PGK-GFP expression shuttle vector. The rAAV particles were isolated either by the traditional cell lysis freeze-thaw with centifugation method or with the AAVanced Concentration Reagent from the packaging cell media. Equal amounts of rAAV were then added to HEK293 cells to test for infectivity based on GFP expression. Representative images of GFP expressing cells 10 days after infection.
AAVanced rAAV Isolation Works with Multiple Serotypes In Vitro
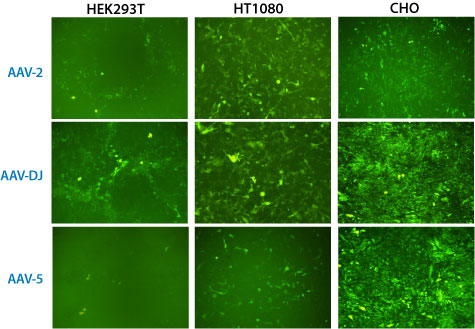
Recombinant AAV was packaged in parallel with three different serotypes: AAV-2, AAV-DJ and AAV-5. The exact same shuttle rAAV vector with a PGK promoter expressing GFP was used for all tests. The media was collected after 48 hours and the rAAV particles in the media were isolated using the AAVanced Concentration Reagent. Each rAAV particle pellet was resuspended in 100ml sterile PBS and then 1ml of the rAAV suspensions were added to three different cell lines (HEK293T, HT1080 and CHO) to validate transduction efficiencies. The infected cells were imaged for GFP expression 6 days after addition of the isolated rAAV particles.
AAVanced rAAV Particles Work In Vivo
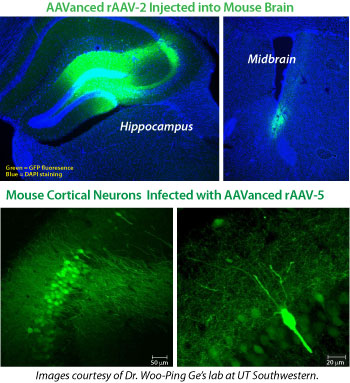
Shown in the upper right panels are representative images of hippocampal and midbrain sections from 6 week old C57 mice injected with AAV virus (ITR-PGK-GFP-ITR) concentrated using AAVanced Concentration Reagent. Approximately 1.5ml of concentrated AAV virus (AAV-2 serotype) was delivered to hippocampal and midbrain regions by stereotaxic injection. Three weeks after virus injection, the animals were perfused with paraformaldehyde, fixed, and brains specimen were sectioned to 40 micron slices before visualization for GFP fluorescence.
Shown in the lower right panels are representative images of mouse neurons in cortical sections of 2 month old C57 mice injected with AAV-5 virus (ITR-PGK-GFP-ITR) concentrated using AAVanced Concentration Reagent. Approximately 1.5ml of concentrated rAAV-5 virus was delivered to cortical regions by stereotaxic injection. Two weeks after virus injection, animals were perfused by paraformaldehyde, fixed and brains were sectioned to 70 micron slices before visualization for GFP fluorescence.
References
- Doria M et al. AAV2/8 vectors purified from culture medium with a simple and rapid protocol transduce murine liver, muscle, and retina efficiently. Hum Gene Ther Methods. 2013 Dec;24(6):392-8.
- Lock M et al. Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther. 2010 Oct;21(10):1259-71.
- Petrs-Silva H, Linden R. Advances in recombinant adeno-associated viral vectors for gene delivery. Curr Gene Ther. 2013 Oct;13(5):335-45.
- Vandenberghe LH et al. Efficient serotype-dependent release of functional vector into the culture medium during adeno-associated virus manufacturing. Hum Gene Ther. 2010 Oct;21(10):1251-7.
- Vasileva A, Jessberger R. Precise hit: adeno-associated virus in gene targeting. Nat Rev Microbiol. 2005 Nov;3(11):837-47.

