Plasma/Serum Cell Free Circulating DNA (cfc DNA, ccf DNA) Purification Micro Kit
| Specifications | |
|---|---|
| Product Category: | Circulating DNA Purification |
| Sample Type: | Plasma/Serum |
Product Description
- For rapid and simple purification of all sizes of circulating DNA from plasma and serum samples
- Isolate viral and bacterial DNA
- Versatile plasma and serum input volumes (10 µL - 200 µL)
- Concentrate circulating DNA into a flexible elution volume ranging from (25 µL - 50 µL)
- Isolate inhibitor-free cell-free circulating DNA
- Purify high-quality DNA in 15-20 minutes
- Compatible with Streck Cell-Free DNA BCT® Tubes
- Purification is based on spin column chromatography that uses Norgen’s proprietary Silicon Carbide resin separation matrix
This kit provides a fast, reliable and convenient spin column method for the isolation of high quality, high purity and inhibitor-free cell-free circulating DNA (cfc-DNA) from small fresh or frozen plasma/serum sample volumes ranging from 10 μL up to 200 μL.
The kit is designed to isolate all sizes of cfc-DNA from either fresh or frozen plasma/serum samples and the purified DNA is eluted into a flexible elution volume ranging from 25 µL to 50 µL. The purified plasma/serum cfc-DNA is fully compatible with all downstream applications including PCR, qPCR, methylation-sensitive PCR and Southern Blot analysis, microarrays and NGS.
Background
Plasma/Serum cell-free circulating DNA (cfc-DNA) has the potential to provide biomarkers for certain cancers and disease states as well as fetal DNA in maternal blood. Currently, significant advancements are being made in utilizing cfc-DNA as biomarkers for the early diagnosis, prognosis and monitoring of therapy for several cancer types and autoimmune diseases. Cell-free mitocondrial DNA (cfmtDNA) is also under investigation for its clinical significance. This cfc-DNA is usually present as short fragments of less than 1000 bp. In addition, cell-free fetal DNA has been widely used as a non-invasive method for prenatal diagnosis including early identification of fetal sex, genetic studies for families at high risk for inherited genetic disorders, screening for Rhesus factor, screening for aneuploidy and identification of preeclampsia.
Performance Data
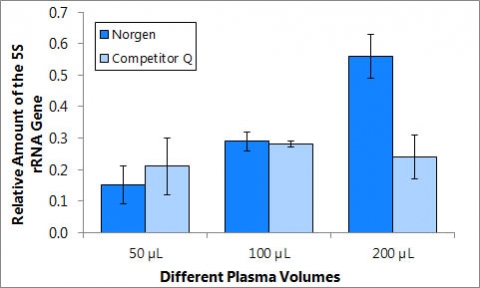
Figure 1. Purification of DNA from different plasma volumes.
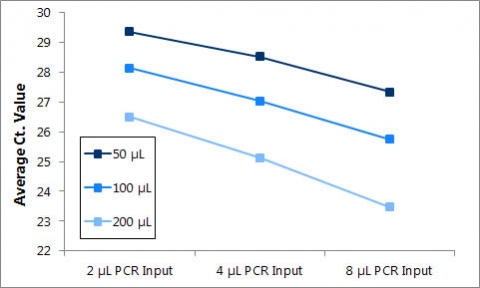
Figure 2. Detection of the human 5S gene
Product Citations
- Yang, X., Tang, Y., Mason, S. D., Chen, J., & Li, F (2016) Enzyme-Powered Three-Dimensional DNA Nanomachine for DNA Walking, Payload Release, and Biosensing ACS Nano
- Catalog Number
55500-NB - Supplier
Norgen Biotek - Size
- Shipping
RT

