CytoSelect™ 24-well Cell Migration Assay (8 µm), Fluorometric
| Specifications | |
|---|---|
| Product Category: | Cell Migration |
| Detection Method: | Fluorometric |
Product Description
- Fully quantify chemotaxis with no manual cell counting
- Measure chemotaxis in less than 6 hours with most cell types
- Membrane inserts are uncoated to allow use with any chemoattractant
Chemotaxis describes the movement of cells toward or away from a chemical stimulus in their environment. Cell chemotaxis plays a pivotal role in the progression of cancer and other diseases.
CytoSelect™ Cell Migration Assays are ideal for determining the chemotactic properties of cells. The 8 µm pore size is suitable for most cell types including epithelial cells, fibroblasts, and cancer cell lines.
Assay Principle
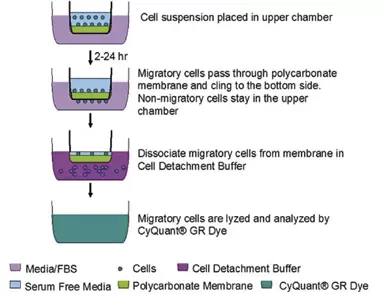
Fig.1. CytoSelect™ Chemotaxis Assay Principle. Migratory cells move through the polycarbonate membrane toward a chemoattractant underneath the membrane inserts.
Supporting Data
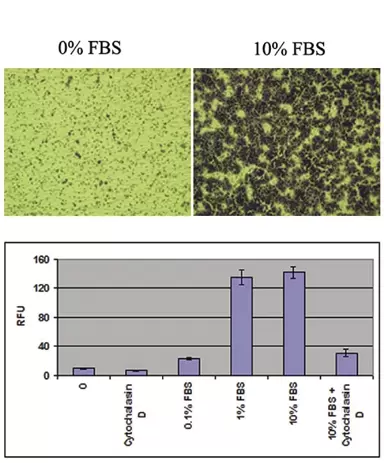
Fig.2. Migration of Human Fibrosarcoma HT-1080 Cells. Cells were seeded at 30,000 cells per well of a 24-well plate and allowed to migrate toward 10% FBS for 4 hours in either the presence or absence of 2µM Cytochalasin D. Migratory cells on the bottom of the polycarbonate membrane were stained (top) and quantified in a fluorescence plate reader (bottom).
Product Citations
- Morgillo F, et al. (2017) Phosphatidylinositol 3-kinase (PI3Kα)/AKT axis blockade with taselisib or ipatasertib enhances the efficacy of anti-microtubule drugs in human breast cancer cells. Oncotarget. doi: 10.18632/oncotarget.20385. eCollection 2017 Sep 29.
- Ibrahim, S.A. et al. (2016) Cancer derived peptide of vacuolar ATPase 'a2' isoform promotes neutrophil migration by autocrine secretion of IL-8. Sci Rep. 6:36865.
- Banerjee, D. et al. (2015) Notch suppresses angiogenesis and progression of hepatic metastases. Cancer Res. 75:1592-1602.
- Słoniecka, M. et al. (2015) Substance P enhances keratocyte migration and neutrophil recruitment through interleukin-8. Mol Pharmacol. doi:10.1124/mol.115.101014.
- Izhak, L. et al. (2010) Predominant Expression of CCL2 at the Tumor Site of Prostate Cancer Patients Directs a Selective Loss of Immunological Tolerance to CCL2 that could be Amplified in a Beneficial Manner. J Immunol. 184:1092-1101.
- Catalog Number
CBA-101-CB - Supplier
Cell Biolabs - Size
- Shipping
RT

