
Phagocytosis Assays
Quantify phagocytosis with no manual cell counting
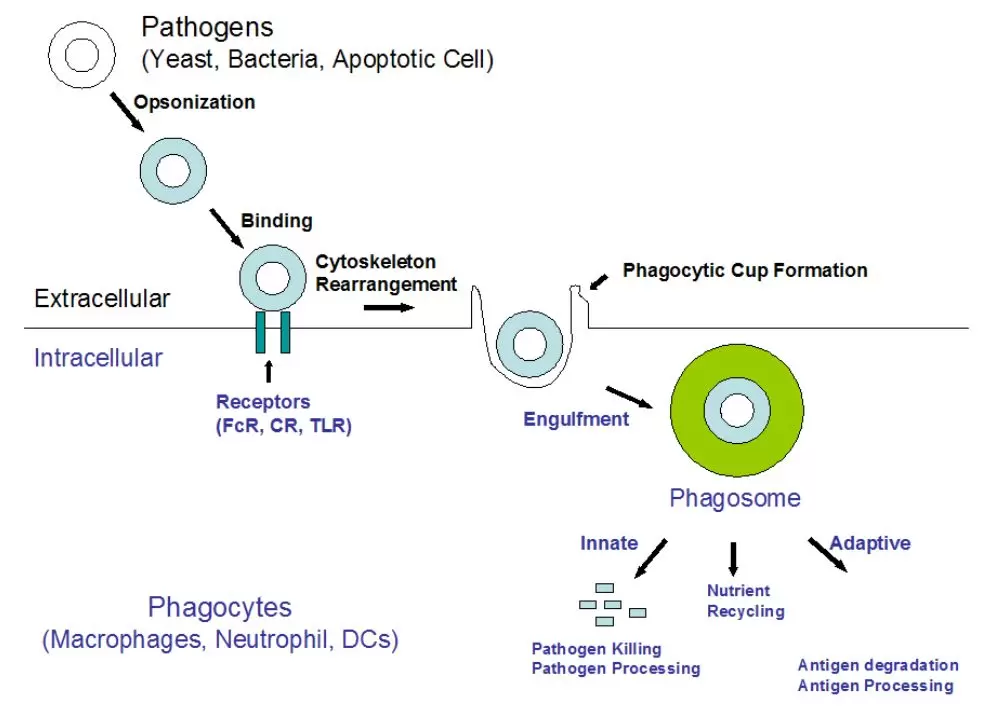
In mammals, phagocytosis by phagocytes (e.g. macrophages, dendritic cells, and neutrophils) is essential for a variety of biological events, including tissue remodeling and the continuous clearance of dying cells. Furthermore, phagocytosis represents an early and crucial event in triggering host defenses against invading pathogens.
Phagocytosis comprises a series of events, starting with the recognition and binding of particles by cell surface receptors, followed by the formation of actin-rich membrane extensions around the particle.
Fusion of the membrane extensions results in phagosome formation, which precedes phagosome maturation into a phagolysosome. Pathogens inside the phagolysosome are destroyed by lowered pH, hydrolysis, and radical attack. These early events that are mediated by the innate immune system are critical for host survival. As a result of this process, pathogen-derived molecules can be presented at the cell surface (antigen presentation), allowing the induction of acquired immunity.
Phagocytosis Processes
Phagocytosis Assays
Traditionally, erythrocytes (red blood cells) are commonly used in phagocytosis assays. For FcR mediated phagocytosis, erythrocytes are first opsonized with serum or IgG before they are added to phagocytes. After removal of non-phagocytic erythrocytes, engulfed erythrocytes are quantitated.
In contrary to conventional phagocytosis assays, the CytoSelect 96-well Phagocytosis Assay does not involve subjective manual counting of erythrocytes. Instead cells are lyzed and detected by the proprietary erythrocyte substrate in a microtiter plate reader. This format provides a quantitative, high-throughput method to accurately measure phagocytosis. The assay provides a robust system for screening Toll-like receptor (TLR) ligands, phagocytosis activators or inhibitors.
Alternatively, the CytoSelect 96-well Phagocytosis Assay (Zymosan particle based) is offered, using Zymosan particles as substrate for quantifying phagocytosis.